PharmaShots Weekly Snapshots (January 01 – January 05, 2023)
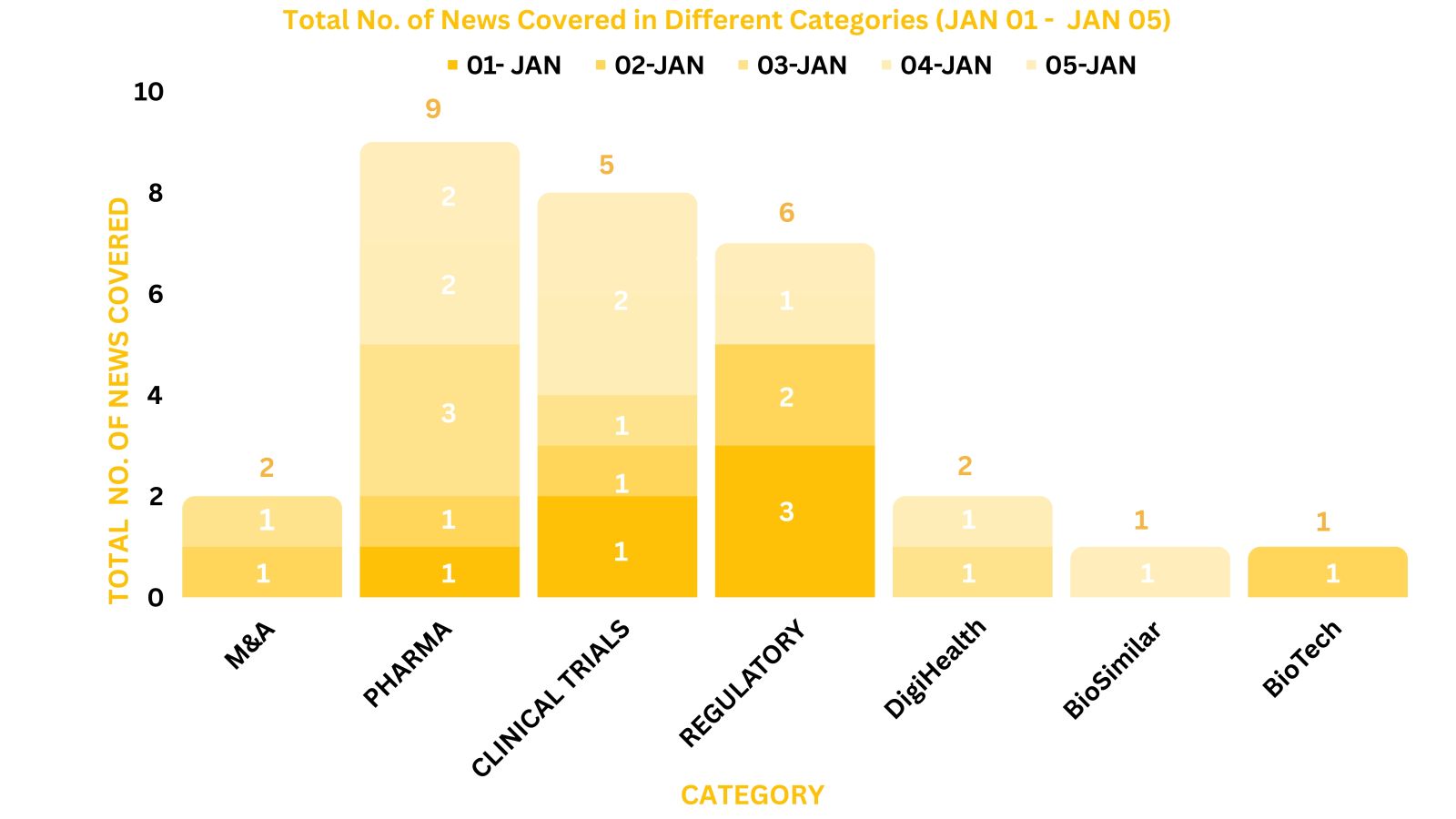
This week PharmaShots’ news was all about the updates on M&A, Pharma, Clinical Trials, Regulatory & MedTech. Check out our full report below:

For an Aggregate of $295M, Roche to Acquire LumiraDx's Point of Care Technology
Read More: LumiraDx
For an Aggregate of ~$185M, Tome Biosciences to Acquire Replace Therapeutics
Read More: Tome Biosciences

HR Pharmaceuticals and Poiesis Medical Sign a Commercialization Agreement for Dual Balloon Catheter Technology in North America
Read More: HR Pharmaceuticals & Poiesis Medical
ANGLE Signs a Contract with Eisai for the Pilot Study Worth $250,000
Read More: ANGLE& Eisai
Voyager Therapeutics and Novartis Sign a Collaboration and License Agreement to Advance Novel Gene Therapies for Huntington’s Disease (HD) and Spinal Muscular Atrophy (SMA)
Read More: Voyager Therapeutics and Novartis
MediLink Therapeutics and Roche Sign a Collaboration and License Agreement to Develop and Commercialize YL211 for Solid Tumors
Read More: MediLink Therapeutics &
Coherus BioSciences Highlights the Availability of Loqtorzi (toripalimab-tpzi) for the Treatment of Nasopharyngeal Carcinoma (NPC) in the United States
Read More: Coherus BioSciences
Elektrofi Signs a Collaboration and License Agreement with Janssen Biotech to Develop Five Programs for Oncology
Read More: Elektrofi
Boehringer Ingelheim Joins Forces with Riboure Pharmaceuticals to Develop RNAi Based Therapies for the Treatment of Liver Diseases
Read More: Boehringer Ingelheim& Riboure Pharmaceuticals
AbbVie Partners with Umoja Biopharma to Develop CAR-T Cell Therapies for Hematologic Malignancies
Read More: AbbVie& Umoja Biopharma
Merck KGaA Signs a Collaboration Agreement with Inspira for the Development and Commercialization of Ompenaclid (RGX-202) to Treat Colorectal Cancer
Read More: Merck& Inspira

The US FDA Imposes Hold on Iovance Biotherapeutics’ Clinical Trial of LN-145 for Non-Small Cell Lung Cancer (NSCLC)
Read More: Iovance Biotherapeutics
Stuart Therapeutics has Reported First Patient, First Visit in the P-III Study of Vezocolmitide for Dry Eye Disease
Read More: Stuart Therapeutics
Innovent Doses First Patient in the P-III (GLORY-2) Trial of Mazdutide (IBI362) in Chinese Adults with Obesity
Read More: Innovent
Longboard Pharmaceuticals Highlights the P-Ib/IIa Results for Bexicaserin (LP352) in Developmental and Epileptic Encephalopathies (DEEs)
Read More: Longboard Pharmaceuticals
Agios Pharmaceuticals Highlights the P-III Results for Pyrukynd (mitapivat) to Treat Non-Transfusion-Dependent Alpha- or Beta-Thalassemia
Read More: Agios Pharmaceuticals
Sitryx Therapeutics’s Partner Eli Lilly Initiates the P-I Study of SIT-011 to Treat Chronic Autoimmune and Inflammatory Diseases
Read More: Sitryx Therapeutics
Lyndra Therapeutics highlight the P-III Results for Risperidone (LYN-005) to Treat Schizophrenia Read More: Lyndra Therapeutics
Tryp Therapeutics Doses First Patient with TRP-8802 in P-IIa Clinical Trial for the Treatment of Fibromyalgia
Read More: Tryp Therapeutics

NeuroBo Pharmaceuticals has Submitted IND Application to the US FDA for Conducting P-I Study of DA-1726 to Treat Obesity
Read More : NeuroBo
Zevra Therapeutics Reports Resubmission of Arimoclomol’s NDA to the US FDA for Treating Niemann-Pick disease Type C (NPC)
Read More: Zevra Therapeutics
Silo Pharma and Sever Pharma Reports Regulatory Approval to Conduct the Development of Ketamine Implant for Fibromyalgia
Read More: Silo Pharma & Sever Pharma
The US FDA Grants aNDA Approval to Loteprednol Etabonate Ophthalmic Suspension
Read More: Lupin
AstraZeneca and Sanofi’s Beyfortus Receives NMPA’s Approval for the Prevention of Respiratory Syncytial Virus (RSV) Disease in Infants
Read More: AstraZeneca
The Clinical Hold Placed by the US FDA on Innate Pharma's Clinical Evaluation of Lacutamab to Treat Sezary Syndrome is Lifted
Read More: Innate Pharma
Health Canada Approves Pfizer’s Beqvez (fidanacogene elaparvovec) for the Treatment of Hemophilia B
Read More: Pfizer

The US FDA Approved AnX Robotica’s ProScan as an AI Assisted Reading Tool for Small Bowel Video Capsule Endoscopy
Read More: AnX
Alex Therapeutics Highlights the Clinical Trial Results for Almee to Treat Anxiety Associated with Pulmonary Fibrosis (PF)
Read More: Alex Therapeutics

Glenmark has Launched Lirafit (Biosimilar, Liraglutide) in India for Type 2 Diabetes Mellitus
Read More: Glenmark

Microbot Medical Reports GLP Pivotal Pre-Clinical Study Results in Porcine Model
Read More: Microbot
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.